Biopact Cellular Transport (Biopact CT) Transfection Technology
Driving down the time and cost of cell R&D and ManufacturingThe Problem
- A critical step in cell engineering is intracellular delivery of genetic material (Transfection)
- Complexities related to Transfection are a major contributor of time and expense to R&D and Manufacturing
- Major issues with current transfection tools that contribute to time and expense:
- Cytotoxicity and cell death
- Low or inconsistent transfection success rate
- Variable effectiveness by cell and donor
- Todays transfection methods are inefficient, lack standardization, are not scalable and therefore time consuming and very costly
Unmet need for new and improved transfection technology

Industry Insights
Research & Developers and Cell Manufacturers work with many combinations of cell and gene therapies and each project requires a different approach
It is estimated that gene vectors may contribute 30% or more to the cost of manufacturing ($150,000 +/- estimated COGS) and R&D
We need to standardize as much of the process as possible in order to increase efficiencies and decrease time and costs of R&D and Manufacturing
The industry is looking to develop more enabling technologies including platform-oriented processes
More standardization is required to control costs, maintain margins and make cell therapies more accessible to patients
We need a transfection technology that is platform orientated, scalable and provides more efficiencies and standardization
http://www.pmlive.com/pharma_intelligence/Cell_and_gene_therapy_1278537
The Solution
Introducing a new, non-biologic cellular transfection technology platform called MGMR™
We believe MGMR™ is:
- More efficient and effective
- Applicable to more cell therapies than any other tool currently available
- Does little to no damage to cells during the transfection process
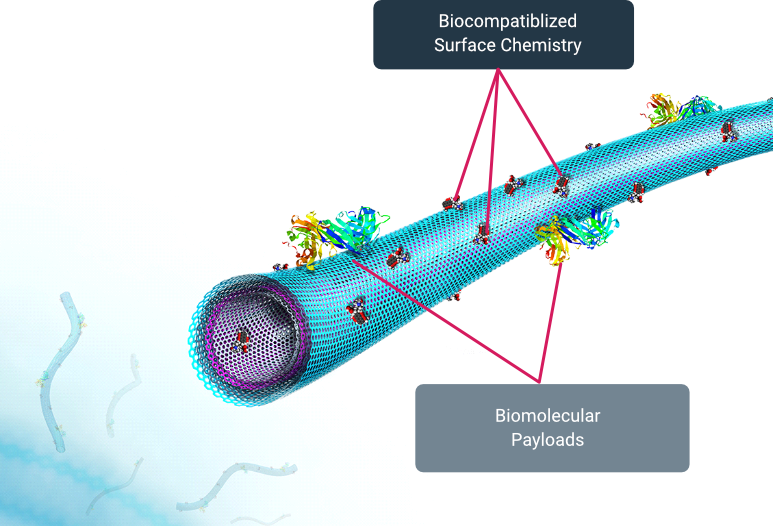
A transfection technology with more potential for standardization and scale with cell therapy than any other transfection technology currently on the market
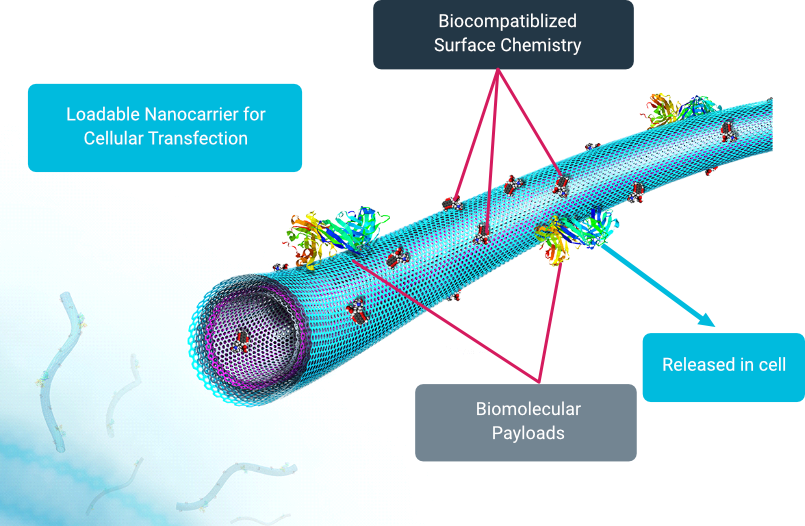
Attributes of MGMR™
- Inert
- Non-biologic
- Non-toxic
- Enters through endocytosis
- Simple to load/unload
- Maintains cell proliferation
- Cargo agnostic
- Does not alter molecular cargo
- Scalable
No single transfection technology has so much potential to influence efficiency, scale, standardization and drive down R&D and Manufacturing costs