Human stem cells cultured with a range of MGMR™ concentrations do not show reduced viability relative to growth media.
Cells treated with a range of concentrations of MGMR™ do not show evidence of chromosomal aberration relative to positive control.
In neoplastic transformation analysis, MGMR™ was not shown to significantly transform cells to soft-agar proliferating colonies relative to positive control.
PTK2 cells (kidney epithelium)
Intracellular distribution
- In eukaryotic cells, MGMR™ localizes within the nucleus and actin cytoskeleton
Cellular uptake produces no evidence of cytotoxicity
Potential for delivery of:
- Genetic materials
- Antibodies
- Proteins
- Enzymes
- Other membrane-impermeable molecules
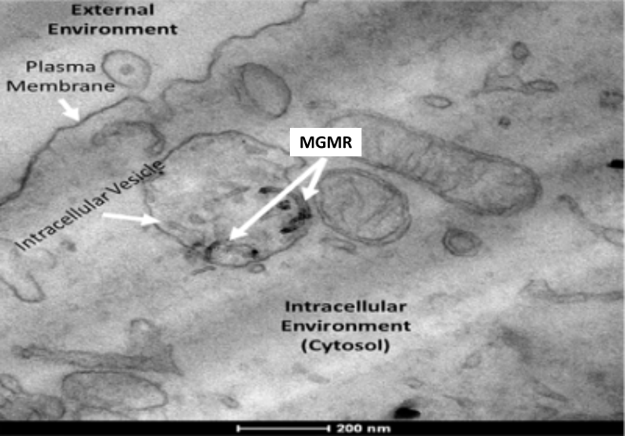
Active transport of MGMR™ offers the potential for intercellular delivery of diverse types biomolecules which does not damage plasma membrane integrity and reduces cytotoxicity.

Mechanism of MGMR™ (CNT) Internalization:
- MGMR™ Vehicles dispersed in the extracellular space are encapsulated within intracellular vesicles,
- Vesicular trafficking into cellular interior,
- Vesicular plasma membrane breakdown and release of vehicles into cytosol.
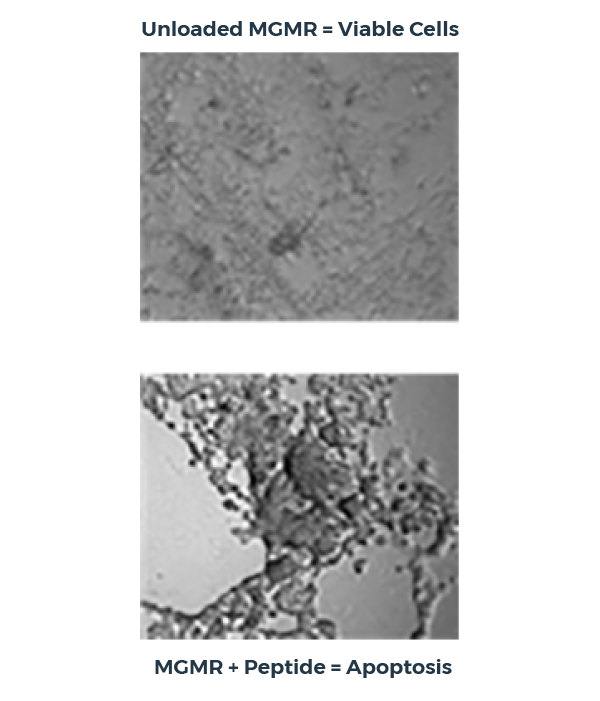
Intracellular peptide transport with MGMR™
In this peptide delivery study, MGMR™ binds a pro-apoptotic peptide with high affinity, transports it across the plasma membrane, and releases it within the cytoplasm, triggering apoptosis. Critically, unloaded MGMR™ does not reduce cellular viability and the peptide alone demonstrates no significant effect.
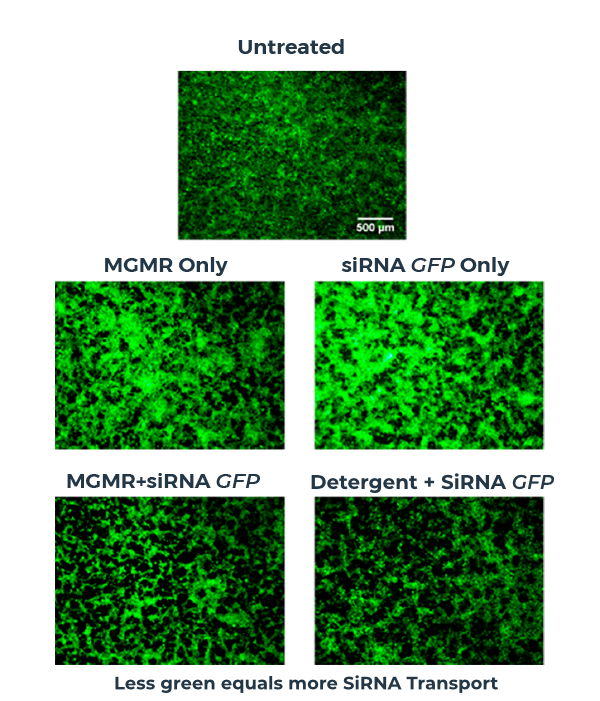
MGMR™ enables efficient intracellular transport of siRNA
In this siRNA delivery study, siRNA-loaded MGMR™ is readily internalized by cells, ferrying siRNA across the plasma membrane and delivering the payload into the cytosol. Efficiency of siRNA delivery is evaluated by mRNA-level expression of the gene of interest.
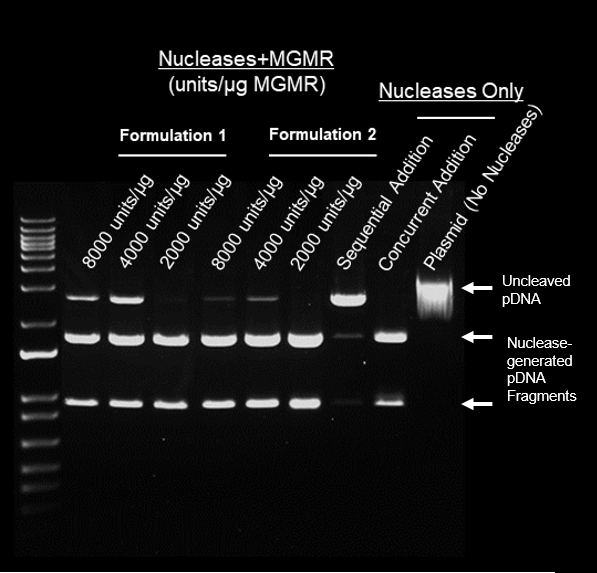
Protocol
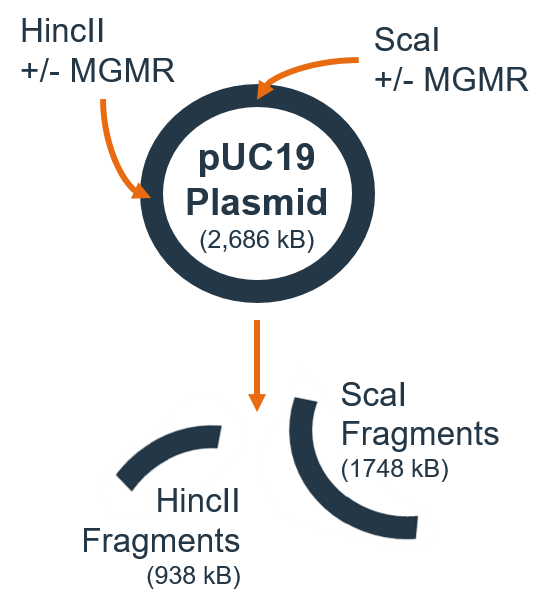
- Scal + MGMR™ incubated with 1ug plasmid for 15 min at 37°C, followed by Hincll + MGMR™ for additional 15 min at 37°C
- Electrophoresis (70 min) – 1% agarose
Results
- Individual nucleases loaded on MGMR™ can cleave pDNA in series
- MGMR™ preserves nuclease activity and demonstrates loading density-depending pDNA cleavage
- Sequential addition of “free” nucleases without MGMR™ produces very low-efficiency pDNA cleavage
- Formulation 2 demonstrated greatest efficiency for serial plasmid cleavage relative to Formulation 1 and sequentially added free nucleases
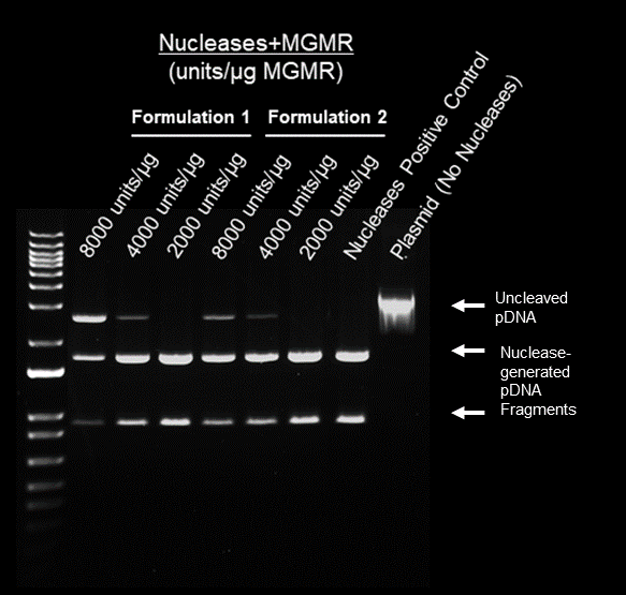
Protocol
- Nucleases + MGMR™ incubated with 1ug plasmid for 15 min. at 37°C
- Electrophoresis (70 min) – 1% agarose
Results
- Enzyme loading on MGMR™ preserves activity of nucleases
- Cutting efficiency of nucleases + MGMR™ relates to loading density (unites/ug)
- Formulations 1 and 2 demonstrate most efficient pDNA cleavage at 2000 units/ug MGMR™

Development of medical-grade, discrete, multi-walled carbon nanotubes as drug delivery molecules to enhance the treatment of hematological malignancies
Burkitt’s lymphoma is an aggressive B-cell derived non-Hodgkin lymphoma. Doxorubicin (Dox) constitutes a common therapy, but features numerous side effects, including significant weight and hair loss, myelotoxicity, cardiovascular damage and treatment-related leukemia. Tumor-specific delivery holds great promise for mitigating adverse side effects while maintaining therapeutic effectiveness of Dox. Carbon nanotubes (CNTs) are highly-prospective drug delivery vehicles due to their unique architecture, high drug-loading surface area and tunable chemistry. Unfortunately, in vivo toxicology, elicited by poor physiological solubility/clearance and high residual contaminants, has prohibited clinical translation of CNTs. XACT™ is a novel drug delivery technology which overcomes these obstacles by utilizing purified, discrete CNTs (dCNTs), bio-compatibilized and highly stable in vivo.
To evaluate long-term stability of dCNTs in physiological conditions, zeta potential (-40 to -50 mV) analysis were performed in ionic, high-serum solutions. No toxicity of dCNTs was observed across multiple cell types in vitro or in murine in vivo models, establishing a high MTD of 70 mg/kg for IV administration. Biodistribution studies (34 days) were conducted in female BALB/C mice using radioisotope-labeled dCNTs. Approximately 60% of injected dCNTs were retained in bone, while the remaining fraction demonstrated high-tolerability and clearance from various organs. Critically, in vitro drug delivery studies showed that dCNT endocytosis is non-cytotoxic and unloaded cargos retain functionality.
We evaluated the considerable potential of dCNTs for bone-specific drug delivery in a murine Raji-Burkitt’s lymphoma model. Whole body bioluminescence imaging revealed that, similarly to those treated with free Dox, groups receiving the Dox-loaded dCNTs showed greatly reduced tumor burden compared to untreated. However, the group receiving Dox-loaded dCNTs also demonstrated increased survival compared to free Dox. Furthermore, typical murine indications of Dox toxicity, characterized by rapid weight loss or drug-related mortality, were not observed in groups receiving Dox-loaded CNTs indicating dCNT’s ability to mitigate negative side effects while retaining efficacy.
In summary, XACT™ dCNTs are a highly-stable, nontoxic and versatile vehicle, featuring a promising capability for targeted delivery to bone tissue. This novel technology provides considerable clinical potential as a therapeutic delivery mechanism for diseases of bone and bone marrow, but also for osteoregenerative applications.
| Location | Conference | Date |
|---|---|---|
| London, UK | Advanced Therapies & Regenerative Medicine Congress | May, 2019 |
| Boston, USA | 7th Immuno-oncology Summit | August, 2019 |
| Amsterdam, NL | Cell Therapy Manufacturing & Gene Therapy Congress | December, 2019 |