The Process of Electroporation
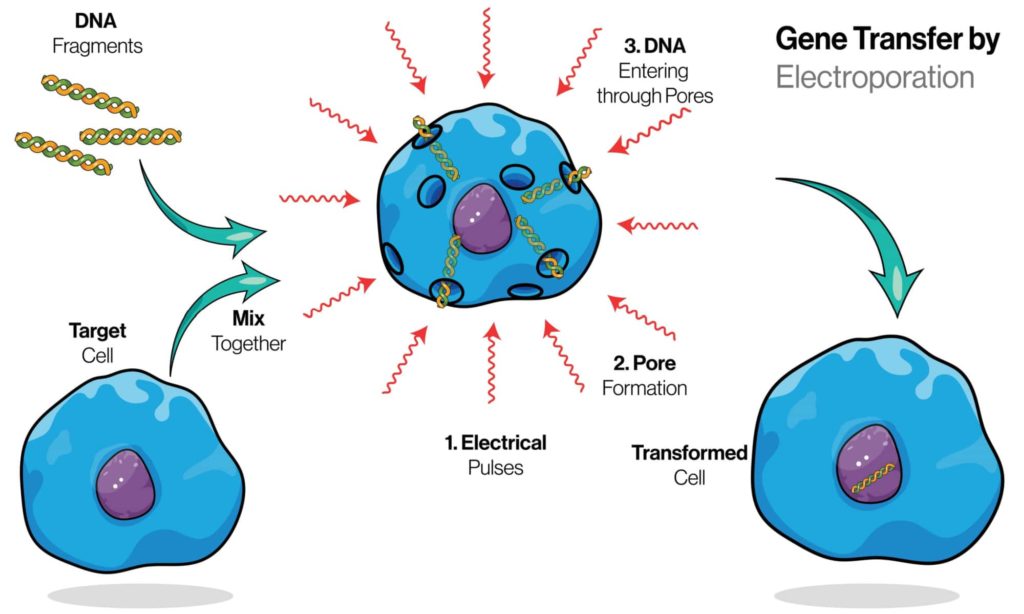
Electroporation is a technique for introducing molecules into cells using an electrical field. Electroporation is often used to introduce DNA or RNA into a cell, but it can also be used to insert protein and small molecules. For example, electroporated DNA can be used as a vaccine or to deliver genes into yeast cells for the expression of proteins that are otherwise not produced by these organisms.
In this blog post, we will discuss the steps involved in the electroporation process along with its applications.
What is produced from electroporation?
Electroporation is an invasiveness-reducing technique for introducing molecules into cells using an electrical field. It is used to introduce DNA, RNA, and siRNA into cells as well as plasmid DNA (in this case, it’s called electrotransformation).
How does electroporation work?
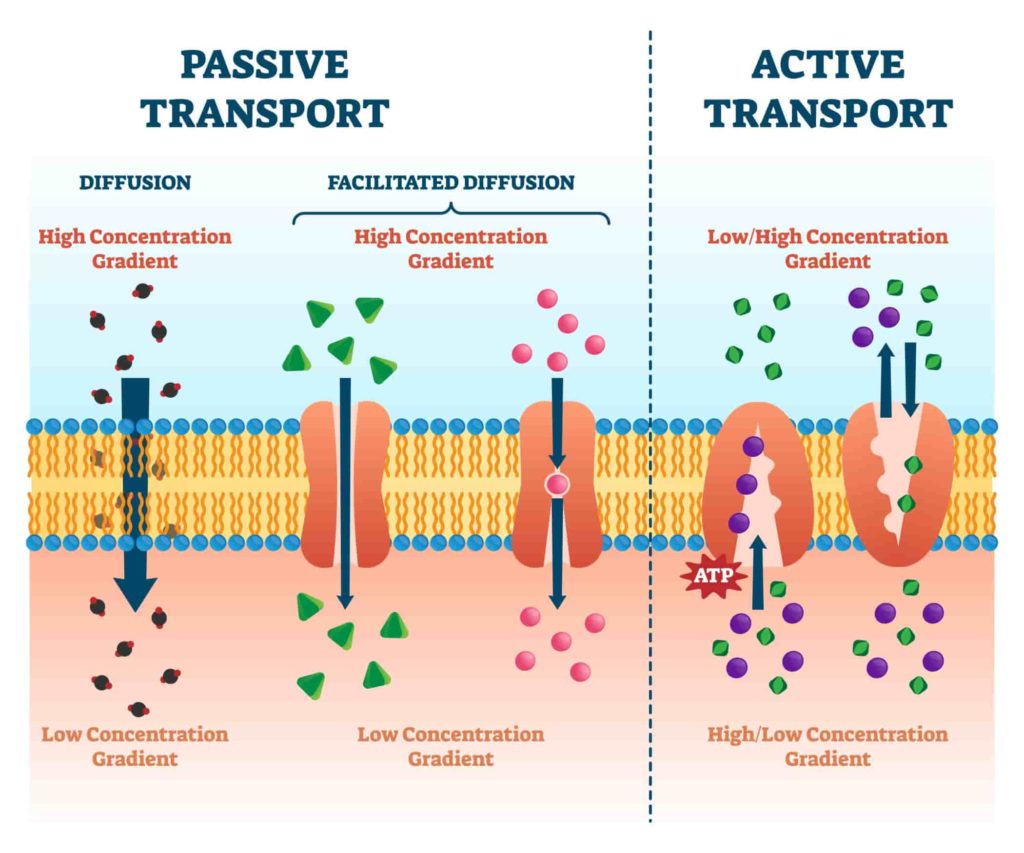
Electroporation is a technique for introducing molecules into cells using an electrical field. The electric field opens the cell membrane to allow the molecules to enter, but only in a tiny area around where the electric field is applied. This will enable you to introduce substances into specific parts of your cells with great precision.
6 Steps to the Electroporation Process
Electroporation is a process that uses a combination of electrical and chemical forces to create temporary pores in the cell membrane. The pores, which are created when an electric field is applied across the cell’s membrane, allow for substances to enter the cell.
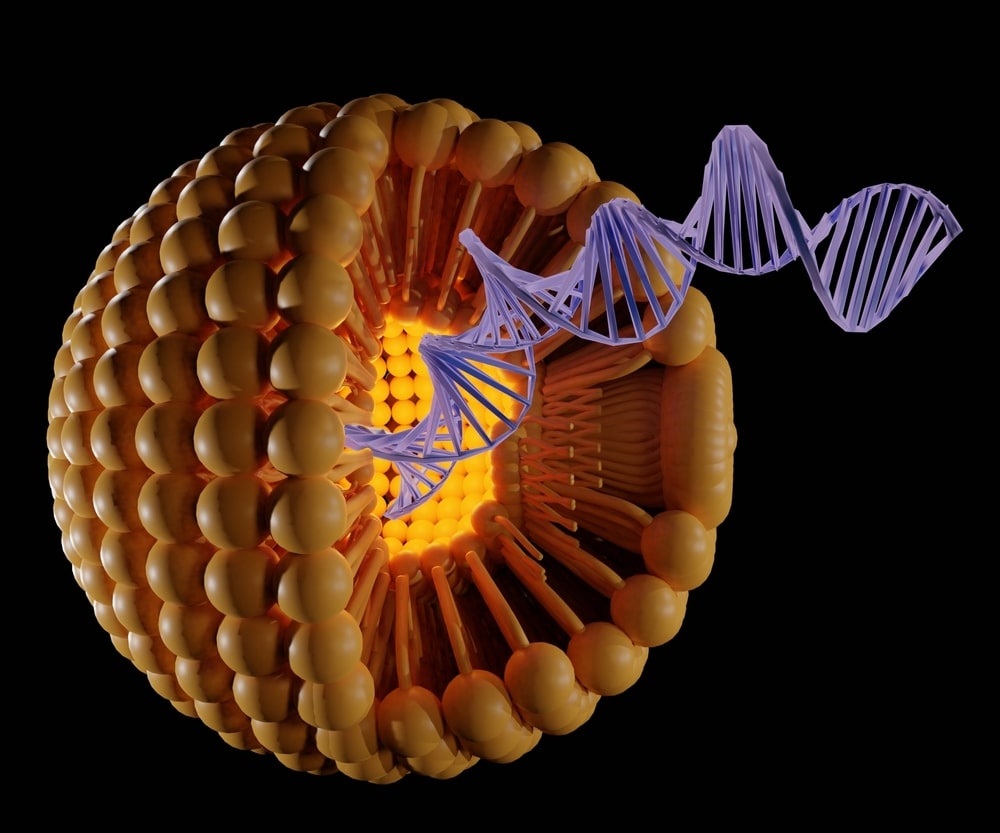
Electroporation can be used to introduce DNA, RNA, or siRNA into cells. The procedure uses a mild electric field to create pores in the cell membrane and enable the introduction of molecules.
In the most basic terms, the electroporator device applies an alternating current at a low voltage (< 3000V). The electric field causes the cell membranes to become porous, allowing entry of DNA and other large molecules which otherwise would not pass through the membrane due to their lipid composition and size (about 4 nm).
Here are six steps to the electroporation process:
Collect and prepare the cells you want to transfect.
You should use an aseptic technique when collecting and preparing cells for electroporation. This means that you should avoid contaminating the cells with anything that isn’t sterile. You should also work in a clean, dry environment free of dust or other contaminants so that your cells remain uncontaminated. If you’re working with animal models, it is important to use anesthetics during this process in order to keep animals from experiencing pain or distress.
Sterile materials are critical for the success of your electroporated transfection experiment because they help ensure that everything stays clean and safe for your cells during the collection and preparation steps. You can purchase sterile gloves at most pharmacies or online; however, contact us if you need help acquiring them!
Add the appropriate reagents (e.g., DNA, RNA, siRNA) to the cell solution.
Association of plasmids
Add the appropriate reagents (e.g., DNA, RNA, siRNA) to the cell solution.
DNA and RNA are added to a culture of cells in order to introduce additional genetic material into the cell nucleus. The process is called transfection or transformation. The amount of DNA or RNA used depends on several factors:
- The type and variant of the target cell line being used; some cells take up more DNA than others do
- Whether you are using a plasmid or a vector to deliver your gene(s) into the cell
- What kind of transfection method you will use (e.g., electroporation vs lipofection)
Apply a short electric pulse to your DNA/RNA-cell mixture.
Once you have completed the above steps, there is one final step. You will apply a short electric pulse to your DNA/RNA-cell mixture. It is important that you use the correct parameters for your cell type and voltage level when doing this step.
The best way to determine what these parameters are for your specific situation is to do some research on reputable sources like PubMed or Wikipedia. Once you’ve decided on an appropriate voltage level, length of time, and frequency (or current), plug it all into your electroporation machine and apply it to the sample!
Incubate the cell mixture at 37°C for about 3 hours.
Incubate the cell mixture at 37°C for about 3 hours. Incubation time depends on the cells and the DNA you are using, so it’s best to consult with your instructor or lab manual if you’re unsure of how long to incubate. The incubation should be done in a humidified chamber set at 37°C.
Analyze the gene of interest and measure expression.
The gene of interest is sequenced and the expression level is determined by measuring the abundance of mRNA using qPCR. Fluorescent reporter genes such as GFP allow you to visualize cells and quantify protein levels, while fluorescent markers can be used to track electroporated cells in culture. The marker can also be an enzyme that catalyzes a reaction when it binds a substrate or reacts with another molecule.
We hope that this post has been helpful in understanding the steps involved in the electroporation process. If you would like to learn more about MGMR technology, or how it can be applied in emerging medical treatments., feel free to check out our blog, or contact BioPact.